Data of Ontozry use in real-world setting: early vs. late use
Second interim analysis of the BLESS study: efficacy and safety of add-on cenobamate in people with focal onset seizures

Effectiveness and safety of adjunctive cenobamate in people with focal-onset epilepsy: Interim results after 24-week observational period from the BLESS study 1
Despite the variety of antiseizure medications (ASMs) and non-pharmacological treatments available, around 30% of people with epilepsy (PwE) continue to experience seizures.1 In most current real-world studies, the average number of ASMs administered before starting cenobamate was high. Nevertheless, increasing real-world evidence is highlighting the benefits of initiating cenobamate at an earlier stage.1
In this context it is important to highlight the “Cenobamate in Adults with Focal-Onset Seizures” (BLESS) study; a multicenter, observational, ambidirectional# trial designed to assess the real-world effectiveness and safety of cenobamate when added to other anti-seizure medications (ASMs) in patients with uncontrolled focal epilepsy.1 The study, which aims to involve 50 Italian centers specialized in epilepsy management and that started on January 24th, 2023, is still ongoing. It includes two planned interim analyses, and this article will focus on the second.1
The second interim analysis aimed to assess outcomes at two distinct time points—12 and 24 weeks after the initiation of cenobamate therapy—in 388 participants.1 Additionally, it includes subgroup analyses, comparing patients with 2 to 3 prior ASMs (early cenobamate users, n=76) to those with more than 3 prior ASMs (late users, n=312).1
Second interim analysis of the BLESS study: main objectives
- Determine the intra-patient percent change and the rates of ≥50%, ≥75%, ≥90% and of 100% reduction in monthly seizure frequency from the pre-treatment baseline over 24 weeks of treatment with cenobamate in early and late users;1
- Evaluate the safety of cenobamate as an adjunctive treatment.1
BLESS Methodology
- Population: male or female adults (age ≥18 years) with uncontrolled focal epilepsy despite treatment with at least 2 ASMs before cenobamate initiation;1
- Duration of cenobamate treatment: at the time of enrollment, all participants were treated for at least 12 weeks (and no more than 52 weeks) with add-on cenobamate;1
- Cenobamate's dosage: at enrollment, the patients should have completed the titration period up to the initial recommended target daily dose of 200 mg;1
- Data collection: data from the index date (the date of cenobamate initiation) to the enrollment have been collected retrospectively. After enrollment, the data collection was prospective for up to 52 weeks. Subgroup analysis was performed in subjects with 2 to 3 previous ASMs (early users) and those with >3 previous ASMs (late users).1
Key Findings
Effectiveness of adjunctive cenobamate in overall population (n=388): reduction in monthly seizure frequency1
- At 24-week follow-up respect to the baseline, the median intra-patient percentage reduction in monthly seizure frequency was 59.9% (−87.3 to −19.2);1
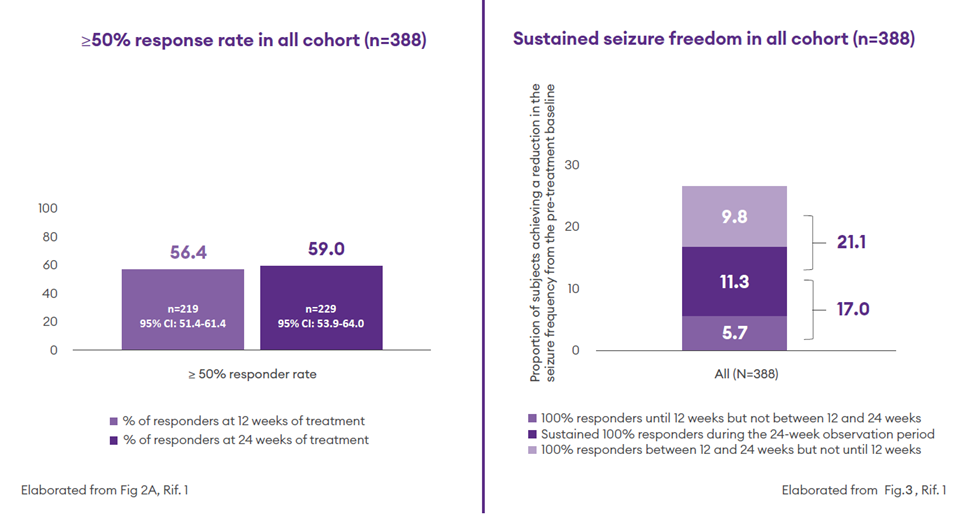
- At 24 weeks, 59% of participants (n = 229) had a ≥50% response rate1
- At 24 weeks, 11.3% of participants were seizure-free without interruption through the considered observation period (sustained seizure freedom)1
- The proportion of participants with a 100% response rate was 17% during the observation period from baseline to 12 weeks, and 21.1% between 12 and 24 weeks (new responders after 12 weeks)1
Early use: early users experienced a greater reduction in monthly seizure frequency compared to late users and showed higher initial 100% response rates already at 12 weeks compared to late users1
- In the early user* subgroup the median intra-patient percentage reduction in monthly seizure frequency from baseline to 24-week follow-up was 78.0% (50.0–97.1%); In the late users **this percentage decreased to 55.5 % (16.4–83.2%);1
- At 24 weeks, 76.3% of early users (n = 58) and 54.8% of late users (n=178) had a ≥50% reduction in baseline seizure frequency:1
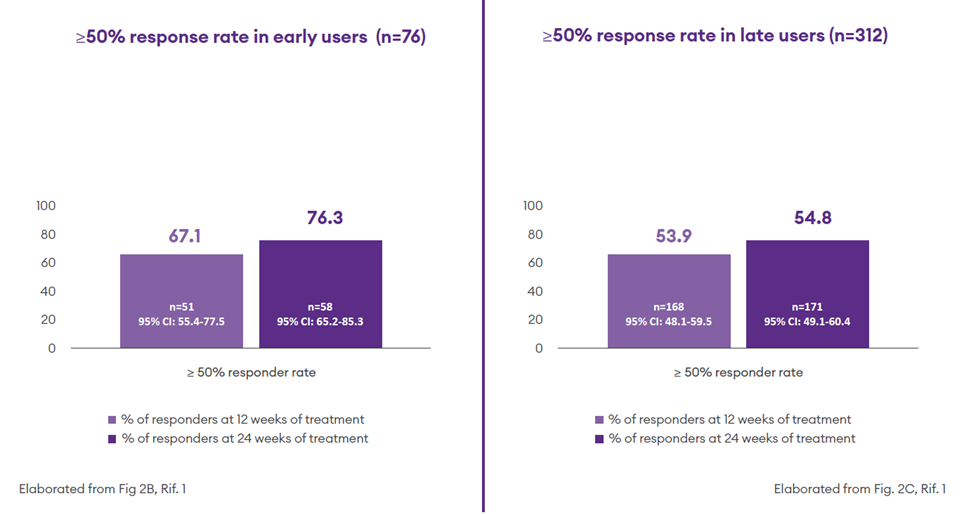
- Sustained seizure freedom was achieved by 22.4% (n=17) of early users and 8.7% (n=27) of late users1
- In the two subgroups the proportion of participants with a 100% response rate was 9.2% and 4.8% from baseline to 12 weeks and 13.1% and 17.6% between 12 and 24 weeks, respectively1

Safety: the safety profile of cenobamate at 24 weeks was predictable and manageable 1
The most frequent adverse events observed were Adverse Drug Reactions (ADRs) to cenobamate.1 The most common ADRs included somnolence, dizziness, and balance disorder, and no serious adverse events (SAEs) were reported.1 After the occurrence of ADRs, 63.5% (n=73) of participants maintained the prescribed dose, and only 5.2% (n=6) permanently discontinued cenobamate.1
Of interest, more ADRs occurred in late** compared to early users*: the total number of ADRs to cenobamate were 110 among late users and 5 among early users. Participants with at least one ADR to cenobamate were 23.4% (n=73) among late users and 5.3% (n=4) among early users. Overall higher number of concomitant ASMs in the late user group may have contributed to this difference.1
Simplicity: adjunctive cenobamate therapy allows the reduction of concomitant ASMs1
The proportion of participants who withdrew at least one concomitant ASM increased in both early and late users, with a more pronounced rise among late users: from 86.8% (n = 66) and 49.0% (n = 151) at baseline to 90.8% (n = 69) and 59.6% (n = 184) at 24 weeks, respectively.1
In this regard, maintaining the effectiveness with the fewest number of ASMs leads to different advantages, such as avoiding overtreatment and drug–drug interactions, reducing the impact on cognition, and improving treatment adherence.1
Ontozry Dose1
Although approximately 80% of participants reached the initial recommended target daily dose of cenobamate (≥200 mg) by 24 weeks, a reduction in monthly seizure frequency was already evident beforehand. This suggests that the drug begins to exert its antiseizure effects early, even before the target dose is attained. These results gain further importance considering that the evaluable subjects in this interim analysis represented a population with difficult-to-treat epilepsy with a median number of 6 previous ASMs and a history of 5 or more prior ASMs in almost 70% of the cases.1
Retention rate1
Nine participants (2.3%, n/N = 9/383) permanently discontinued cenobamate between 12 and 24 weeks from the index date. The primary reasons reported for discontinuation were lack of therapeutic efficacy (n = 3); adverse event (AE, n = 2); lack of adherence (n = 2); seizure frequency unchanged (n = 1), and patient request (n = 1). 1
The reduction of concomitant ASMs was also found to minimize the occurrence of AEs and improve the retention rate.1
Given the importance of full seizure control to achieve a good QoL, these findings underscore the potential of cenobamate as a promising early add on-treatment option to achieve seizure freedom and alleviate the burden of the disease. 1
* Number of previous ASMs used before cenobamate initiation, median (IQR): 3.0 (2.0-3.0)
** Number of previous ASMs used before cenobamate initiation, median (IQR): 7.0 (5.9-9.0)
# Ambidirectional study = a type of cohort study that uses both retrospective (looking back at past data) and prospective (following forward into the future) elements.
FURTHER INFORMATION ON ONTOZRY IN EARLY STAGES (AFTER 2 or 3 ASMs) CAN BE FOUND IN:
- Winter Y. et al. Cenobamate as an Early Adjunctive Treatment in Drug-Resistant Focal-Onset Seizures: An Observational Cohort Study. CNS Drugs. 2024 Sep;38(9):733-742.
- Martinez-Liziana E. et al. Impact of lifetime antiepileptic drug history on cenobamate efficacy in adults with focal epilepsy. Seizure. 2025 Feb:125:94-98.
- Lattanzi S, Dono F, d'Orsi G et al. BLESS Study Group. Effectiveness and safety of adjunctive cenobamate in people with focal-onset epilepsy: Interim results after 24-week observational period from the BLESS study. Epilepsia. 2025;66:2239-2252.
▼Detta läkemedel är föremål för utökad övervakning.
Ontozry® (cenobamat) 12,5 mg odragerad tablett samt 25 mg, 50 mg, 100 mg, 150 mg och 200 mg filmdragerade tabletter. Rx F. ATC-kod: N03AX25 - antiepileptika, övriga antiepileptika. Indikation: Ontozry är indicerat som tilläggsbehandling av fokala anfall, med eller utan sekundär generalisering hos vuxna patienter med epilepsi, som inte kontrollerats tillräckligt trots tidigare behandling med minst två antiepileptika. Kontraindikationer: Överkänslighet mot den aktiva substansen eller mot något hjälpämne, ärftligt kort QT-syndrom. Varningar: Patienter ska instrueras att uppsöka läkare om tecken på självmordstankar/självmordsbeteende uppstår, samt om tecken och symptom på läkemedelsreaktion med eosinofili och systemiska symtom (DRESS) inträffar. Innehåller laktos. Cenobamat kan minska exponeringen av substanser som metaboliseras via CYP3A4, CYP2B6 samt öka exponeringen av substanser som metaboliseras via CYP2C19. Cenobamat rekommenderas inte till fertila kvinnor som inte använder preventivmedel eller vid amning. MAH: Angelini Pharma S.p.A. Lokal kontakt: Angelini Pharma Nordics, nordic.medinfo@angelinipharma.com. Datum för senaste översyn av SPC: 9/2025. För pris och ytterligare information, se www.fass.se.
MAT-DK-0062-P 3.11.2025
 HarmoniaMentis Sverige
HarmoniaMentis Sverige
