THINK …SIMPLICITY
Cenobamate in your clinical practice.

Add-on cenobamate:
Possibility to simplify the treatment by effectively managing concomitant ASMs.
“…It’s just been a trial-and-error process since, which is very frustrating, mainly just because I don’t really know where we go from here?” 1
Since the goal of epilepsy treatment is to achieve seizure freedom with the fewest adverse events (AEs), drug overload reduction seems to be a smart strategy to optimize the efficacy, tolerability, and adherence of a newly initiated ASM. In this scenario, there is an urgent need to develop breakthrough ASMs that increase seizure freedom rates with a balanced tolerability profile, allowing a reduction of co-ASM overload in a rational polytherapy fashion.2
Post-hoc analysis of C021, a large, multicenter cenobamate phase 3 safety study with over 1300 patients showed that concomitant ASM drug load can be reduced in many patients after starting cenobamate therapy regardless of ASM drug class without compromising efficacy3
Information received from cenobamate use in the context of real-world practice gives similar indication; In Italian RWE study (Pietrafusa et al 2023) almost 60% of highly refractory patients with focal onset seizures were able to reduce of withdraw one or more concomitant ASMs.4
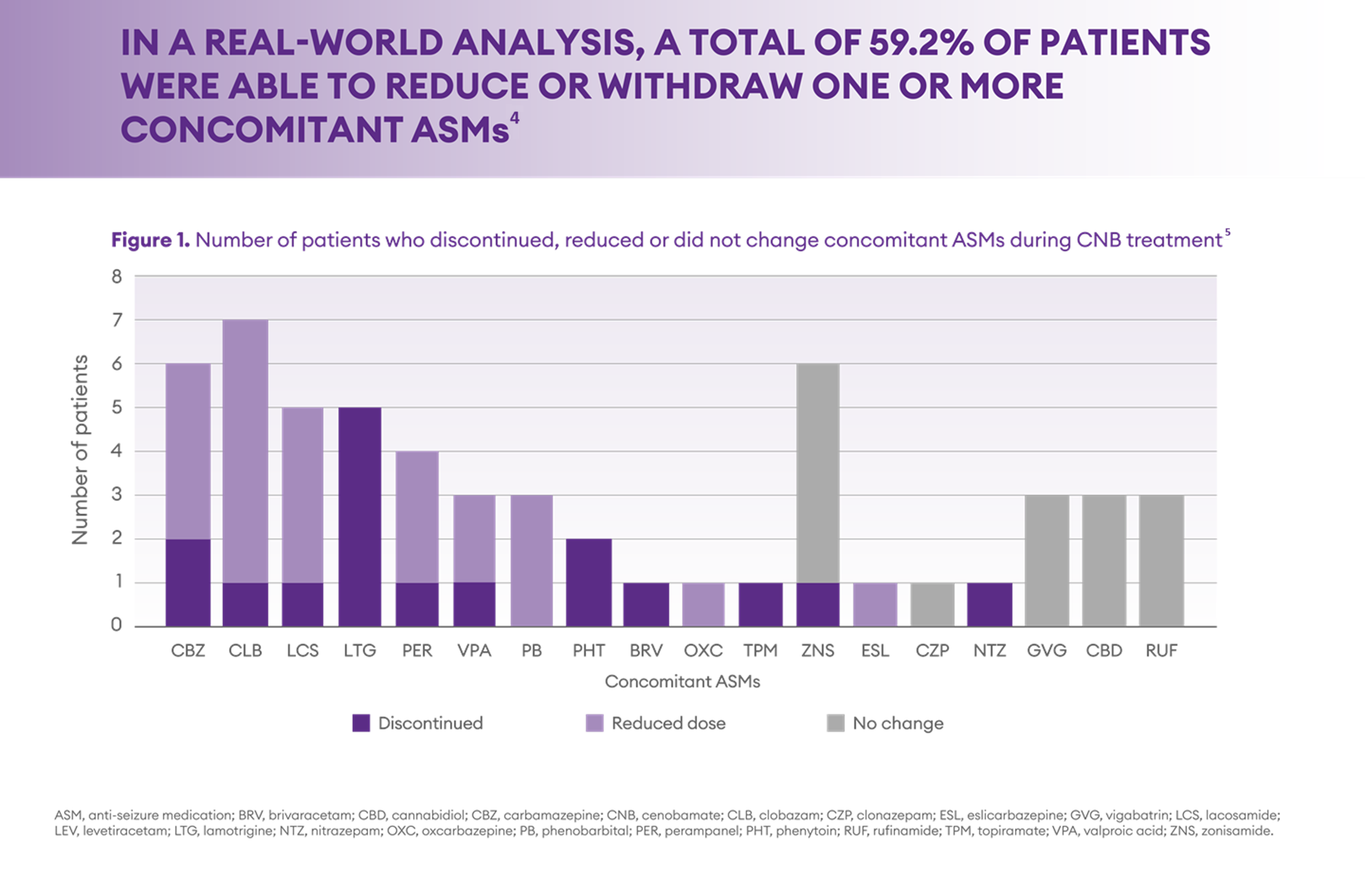
Adapted from Pietrafusa et al. Front Pharmacol 2023
A structured approach to managing concomitant medications when introducing a new treatment for epilepsy patients can help to improve clinical outcomes6. Simplifying the medication regimen by reducing the number of drugs can lead to better adherence. Patients are more likely to consistently take their medications as prescribed if the regimen is less complex and has fewer side effects7.
Cenobamate Dosing and Titration: A Practical Guide
Managing co-medications with cenobamate requires careful patient evaluation. Dose adjustments should therefore be made on a case-by-case basis, bearing in mind the concomitant ASM doses, seizure type and frequency, tolerability to ASMs, and comorbidities.8
We have compiled a couple of practical resources to assist you in your daily clinical practice and to help to maximize the benefits when introducing cenobamate.
On the first link, you can download a simple material that provides practical guidance on dosing and titrating cenobamate for adults with focal-onset seizures. It outlines recommended starting doses, titration schedules, and key considerations for co-medication management to optimize patient outcomes and minimize adverse events:
Secondly, on the link below you can click to a short video where Dr. Patrick House (Epileptologicum Hamburg) gives an insightful overview of the introduction of cenobamate, focusing on its integration with existing medications and strategies for managing interactions with other antiseizure drugs to enhance patient outcomes.
▼ Detta läkemedel är föremål för utökad övervakning.
Ontozry® (cenobamat) 12,5 mg odragerad tablett samt 25 mg, 50 mg, 100 mg, 150 mg och 200 mg filmdragerade tabletter. Rx F. ATC-kod: N03AX25 - antiepileptika, övriga antiepileptika. Indikation: Ontozry är indicerat som tilläggsbehandling av fokala anfall, med eller utan sekundär generalisering hos vuxna patienter med epilepsi, som inte kontrollerats tillräckligt trots tidigare behandling med minst två antiepileptika. Kontraindikationer: Överkänslighet mot den aktiva substansen eller mot något hjälpämne, ärftligt kort QT-syndrom. Varningar: Patienter ska instrueras att uppsöka läkare om tecken på självmordstankar/självmordsbeteende uppstår, samt om tecken och symptom på läkemedelsreaktion med eosinofili och systemiska symtom (DRESS) inträffar. Innehåller laktos. Cenobamat kan minska exponeringen av substanser som metaboliseras via CYP3A4, CYP2B6 samt öka exponeringen av substanser som metaboliseras via CYP2C19. Cenobamat rekommenderas inte till fertila kvinnor som inte använder preventivmedel eller vid amning. MAH: Angelini Pharma S.p.A. Lokal kontakt: Angelini Pharma Nordics, nordic.medinfo@angelinipharma.com. Datum för senaste översyn av SPC: 2/2025. För pris och ytterligare information, se www.fass.se.
- Reeder S, Foster E, Vishwanath S et al. Experience of waiting for seizure freedom and perception of machine learning technologies to support treatment decision: A qualitative study in adults with recent onset epilepsy. Epilepsy Res. 2023;190:107096.
- Rodríguez‐Uranga JJ, Sanchez-Caro JM, Ramchandani RH. Treatment simplification to optimize cenobamate effectiveness and tolerability: A real‐world retrospective study in Spain. Epilepsia Open. 2024 May 27.
- Aboumatar S, Ferrari L, Stern S et al. Reductions in concomitant antiseizure medication drug load during adjunctive cenobamate therapy: Post-hoc analysis of a subset of patients from a phase 3, multicenter, open-label study. Epilepsy Res. 2024;200:107306.
- Pietrafusa N, Falcicchio G, Russo E et al. Cenobamate as add-on therapy for drug resistant epilepsies: effectiveness, drug to drug interactions and neuropsychological impact. What have we learned from real word evidence? Front Pharmacol. 2023;14:1239152.
- Pietrafusa N, Falcicchio G, Russo E et al. Cenobamate as add-on therapy for drug resistant epilepsies: effectiveness, drug to drug interactions and neuropsychological impact. What have we learned from real word evidence?Front Pharmacol. 2023;14:1239152. Supplementary material.
- Perucca, E., & Tomson, T. 2011. The pharmacological treatment of epilepsy in adults. The Lancet Neurology, 10(5), 446-456
- Faught, E. 2012. Adherence to antiepilepsy drug therapy. Epilepsy & Behavior, 25(3), 297-302
- Steinhoff B, Ben-Menachem E, Klein P et al. Therapeutic strategies during cenobamate treatment initiation: Delphi panel recommendations. Ther Adv Neurol Disord 2024, Vol. 17: 1–10
MAT-DK-0022-P Apr 2025
 HarmoniaMentis Sverige
HarmoniaMentis Sverige